

It's just that they don't use that 3d orbital as frequently because once they've reached that noble gas electron configuration, they're stable and don't have an overpowering desire to exceed that octet. So third row elements are similar to second row elements like carbon and oxygen who also want to have an octet, however third row elements aren't limited to only have 8 electrons because of that 3d orbital and can have greater than 8. So third row elements like to have an octet to have an electron configuration similar to a noble gas like argon because it makes them more stable. Only when we start doing the electron configurations of 4 row transition metals like iron and cobalt do we start back filling the 3d orbital. Once those 3s and 3p orbitals are filled, we'll have used 8 electrons. Valence electrons being the electrons that are important to chemical bonding. But if we have an element in the third row (or period) like sulfur or phosphorus, we're only going to fill up the 3s and 3p orbitals in their electron configurations because that's where their valance electrons are. So in the third shell there's s, p ,and d orbitals which, if fully filled, do add up to 18 electrons. Sorry for the long response, but that's the stuff you need to know to do electron configurations well. The order which you fill subshells for more massive elements follows the aufbau principal. The first 1 is the shell number, the s is the subshell the electron exists in, the 1 superscript is the number of electrons in that subshell. Shell numbers are written first, then the subshell as a letter, then the number of electrons in that subshell are written as a superscript to the letter.įor for example neutral hydrogen has the electron configuration of: 1s^(1). Putting all these together you can specify exactly which electron you are referring to in an atom.įor the purposes of electron configuration though, we only care about the principal quantum number (the shell number), the angular quantum number (the subshell or the letter), and the number of electrons in those subshells which are basically a result of the magnetic quantum and spin quantum numbers. This is why orbitals can only hold a maximum of 2 electrons because they can only take on one of two ms values. This tells us why the s subshells only have 1 orbital while p subshells have 3, and the other subshells have the amount of orbitals that they do (and by extension how many electrons they can hold).įinally there is spin quantum number, represented by ms, of which an electron in an orbital can only take on two values: +1/2 or -1/2, broadly referring to the electron spinning clockwise or counterclockwise if you want to think of it like that.

The values of ml in a subshell range from +l to -l. The magnetic quantum number, represented by ml, tells you the specific orbital within the subshell an electron is in.
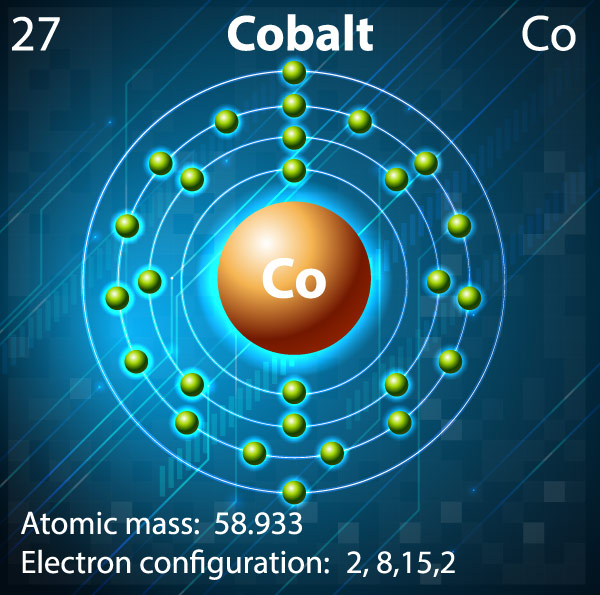
The values of l correspond to the subshell letters, so: l=0 is s, l=1 is p, l=2 is d, and l=3 is f. The subshell tells us broadly the shape the orbitals which hold the electrons take on and these are represented by the letters s, p, d, and f. Electrons in the second electron shell can have a value of l=0 AND 1 since n=2, and so on. So electrons in the first electron shell can only have a value of l=0 since n=1. The allowed values of l in an electron shell are determined by: l = n-1. The angular quantum number tells you the subshell an electron is in within an electron shell and is represented by the letter 'l' often. So elements in the first row of the periodic table have the first electron shell, or n=1, as their valence shell or outermost electron shell. The principal quantum number, often represented by n, is another term for the electron shell and are positive integer values (n=1,2,3,etc.) These electron shell numbers broadly correspond to the period, or row, an element is in.

Due to the Pauli exclusion principal no two electrons can have exactly the same quantum numbers. These include the principal quantum number, the angular quantum number, the magnetic quantum number, and spin quantum number. These regions where we find electrons are represented by the quantum numbers, of which there are four. So electrons exists in clouds in a way around the nuclei of atoms.


 0 kommentar(er)
0 kommentar(er)
